
Should serious adverse events associated with dietary supplements be reported to FDA?Ī. Responsible companies in the industry have fully supported the need for dietary supplements GMPs in order to create a level playing field for companies across the board and help increase consumer confidence in the quality and safety of these products. Firms with less than 500 employees had to be compliant by June 2009, and for small manufacturers that employ less than 20 employees, the compliance date was June 2010. And for large companies – more than 500 employees – the compliance date was June 2008. The GMP rule provided a staggered three-year “phase in” compliance period for manufacturers. In June 2007, GMPs specific to dietary supplements were released from FDA. Responsible companies do abide by GMPs-and many observe procedures which go above and beyond what the current regulations require. Shouldn’t companies have to abide by Good Manufacturing Practices (GMPs)?Ī. Further, DSHEA provided FDA with additional enforcement authority, including the ability to remove from the market products the agency deems unsafe through: 1) an “imminent hazard” clause which permits FDA to immediately remove a product it considers to present an immediate safety concern and 2) a “significant or unreasonable risk” clause that allows removal of a product considered to pose an unacceptable risk of illness or injury. DSHEA specifically reaffirmed the status of dietary supplements as a category of food and created a specific definition for dietary supplements. But the overwhelming majority of dietary supplements are safely used by 170 million Americans annually.Ī. There are provisions under DSHEA that protect consumers from potentially unsafe products. Like food products, dietary supplements do not undergo pre-market approval, but that does not mean that companies don’t do testing, or that products are unsafe. Pre-market approval is not a guarantee of safety as witnessed by those drug products that have been approved by FDA, only to be later recalled due to safety concerns. Without pre-market approval, how do we know these products are safe?Ī. Since the passage of DSHEA, FDA has turned down about half of the New Dietary Ingredient notifications filed.
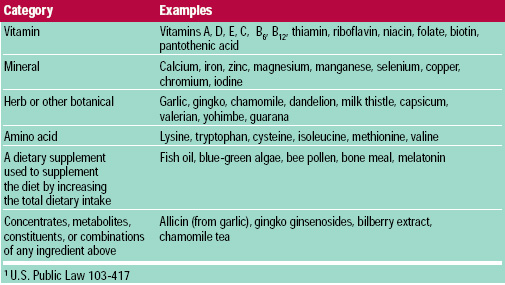
If FDA has any concerns about the ingredient or submitted safety profile, the agency can request more information or deny the product’s entry into the marketplace. If a supplement manufacturer wants to introduce a new ingredient, it must provide FDA with 75 days notice, along with safety information. before passage of DSHEA are “grandfathered” and assumed to have a history of safe use. Under the law, dietary supplements marketed in the U.S. FDA never had pre-market approval over dietary supplements, and DSHEA did not change that fact. Is it true that before DSHEA was passed in 1994, FDA had pre-market approval authority?Ī. If dietary supplements were regulated like drugs, there would likely be no dietary supplement industry and the products that did exist would cost what drugs cost. Virtually all facets of dietary supplement manufacturing, labeling and marketing are covered by extensive regulations issued and enforced by FDA and FTC. Dietary supplements have always been regulated as a category of food in this country, and DSHEA did not change that fact. When critics say dietary supplements are “unregulated,” what they generally mean is that dietary supplements are not regulated like drugs. Why do some people say the industry is unregulated?Ī. The FDA has regulatory authority under the Federal Food, Drug and Cosmetic Act as amended in 1994 by the Dietary Supplement Health and Education Act (DSHEA) and in 2006 by the Dietary Supplement and Nonprescription Drug Consumer Protection Act. The dietary supplement industry is regulated by FDA and the Federal Trade Commission (FTC). Is the dietary supplement industry regulated?Ī. More than 170 million Americans take dietary supplements annually. When used properly, they help promote overall good health and prevent disease. These products are intended to be used as supplements to, not substitutes for, a well-balanced diet and a healthy lifestyle. Dietary supplement products include vitamins, minerals, botanicals, sports nutrition supplements, weight management products and specialty supplements. In the U.S., the dietary supplement industry is a $46 billion 1 industry. Who is the dietary supplement industry?Ī. Learn more about how the dietary supplement industry is regulated by FDA and FTC in CRN's Q&A. International Trade & Market Development.Economic Impact of the Dietary Supplement Industry.Dietary Supplement Health & Education Act (DSHEA).Private Equity Loves Dietary Supplements.CRN Member Company Brands and Branded Ingredients.


 0 kommentar(er)
0 kommentar(er)
